BBC News 3 February 2021 - by Nick Triggle and Philippa Roxby
A vaccine to tackle the coronavirus variants could be ready to deploy by the autumn, the team behind the Oxford-AstraZeneca vaccine says.
Prof Andy Pollard, from Oxford University, said they were already planning to tweak the vaccine.
There is still strong evidence existing vaccines work well against the mutations that have emerged.
Although their overall effectiveness may be weakened a little.
The comments came after results released by the team showed the first evidence the vaccine can reduce the chances of people catching and passing on the virus, which has always been uncertain.
The data, which has not yet been published or reviewed, showed vaccination with the Oxford-AZ jab could cut transmission by up to 67%.
This means the vaccine could significantly slow the spread of the virus, potentially allowing restrictions to be lifted more quickly.
Previous studies have already shown the vaccine is good at preventing serious illness and death from Covid-19 in those given the jab.
Health Secretary Matt Hancock said the results were "absolutely superb" and showed vaccines are "the way out of this pandemic".
But he said the "on-going challenge" would be for vaccine manufacturers to keep up with what the virus is doing.
There is most concern about the South African variant, which shows signs of being able to escape some of the protective effect of the vaccines. There are already signs this has begun circulating in some parts of the UK, prompting surge testing to be introduced into parts of London, Surrey, Kent, Hertfordshire and Southport.
The mutation - called E484K - has also been detected in some of the infections caused by UK strains that are circulating in parts of Bristol and Liverpool.
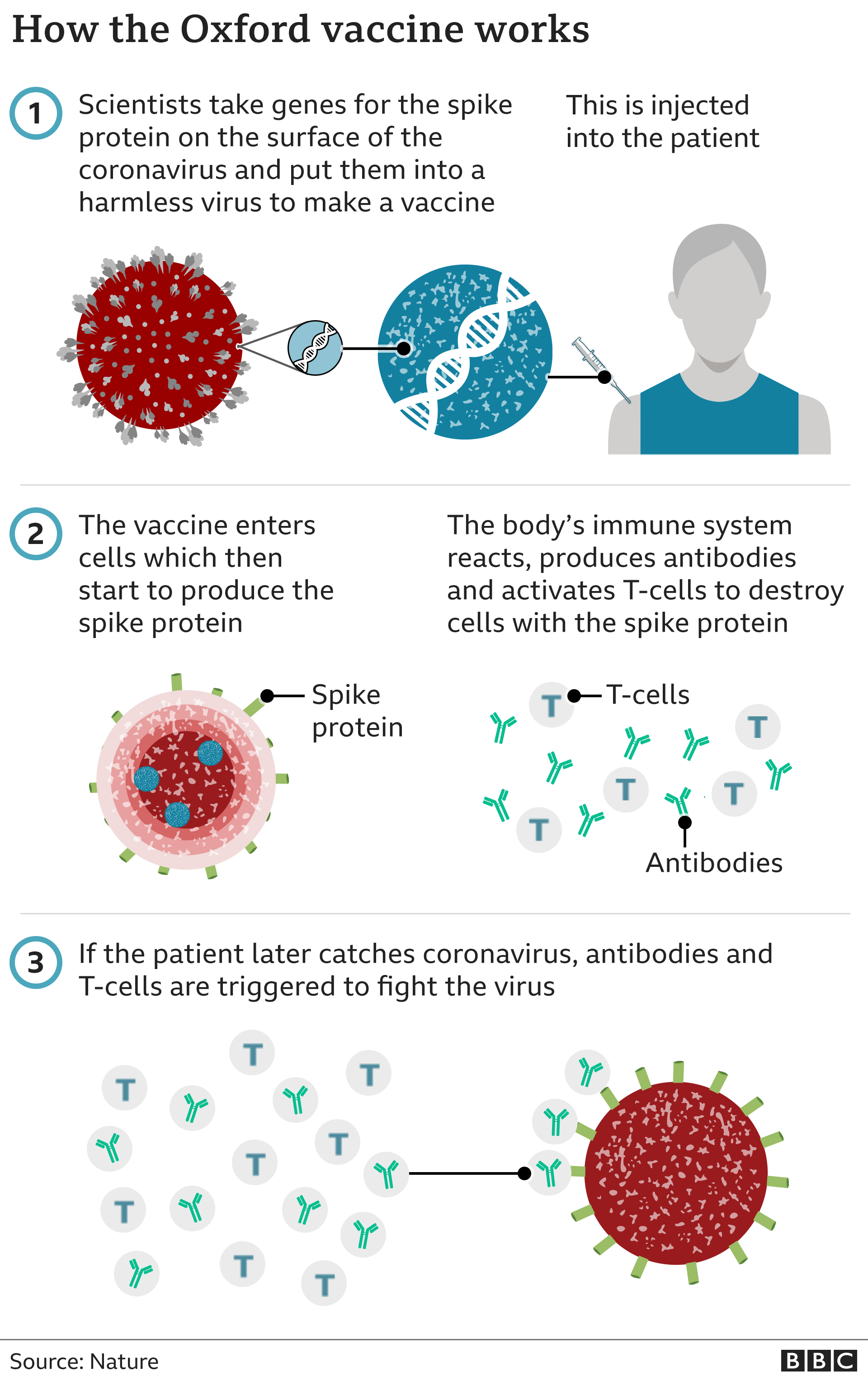
Prof Pollard said his team were already looking at updating the vaccine to make it more effective against the mutations that are being seen.
"I think the actual work on designing a new vaccine is very, very quick because it's essentially just switching out the genetic sequence for the spike protein.
"And then there's manufacturing to do and then a small scale study. So all of that can be completed in a very short period of time, and the autumn is really the timing for having new vaccines available for use," he said.
Sir Mene Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, added: "Our ambition is to be ready for the next round of immunisations that may be necessary as we go into next winter. That's what we're aiming for."
He added the manufacturing process would also be easier as plants would be fully up to speed by then.
The trials that would need to be run are only likely to involve a few hundred people as the team would only need to check safety and that a good immune response is generated by carrying out blood tests.